 I wanted to revisit the Ocean Iron Fertilization idea I mentioned in my last blog post, to run the numbers and see if it's worth the controversy. I found out some interesting things.
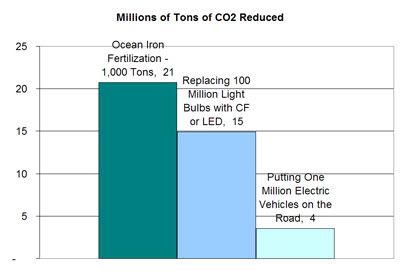
I wanted to revisit the Ocean Iron Fertilization idea I mentioned in my last blog post, to run the numbers and see if it's worth the controversy. I found out some interesting things.I compared three approaches for reducing CO2: (1) OIF, (2) replacing 100 million incandescent bulbs with compact fluorescent or LED bulbs, and (3) replacing 1 million gas-guzzling cars with electric vehicles. The results are surprising. 1,000 tons of iron seeded into oceans beats both other options combined. And that's a small amount considering scientists are thinking about seeding 200,000 tons of iron into the oceans.
How do these compare to the size of the problem at hand. Consider that in 2030 the U.S. will emit 3.3 billion tons of CO2 into the air. So 40 million tons is barely over 1%. Now think that it would take 159,000 tons of iron to sequester 100% of the U.S.'s 2030 CO2 levels, and ocean iron fertilization begins to sound very intriguing, maybe even worth the risks.
Here are the rough calculations I did:
First off, 1,000 tons of iron seeded into algae blooms could sequester roughly 21 million tons of CO2.
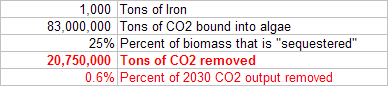
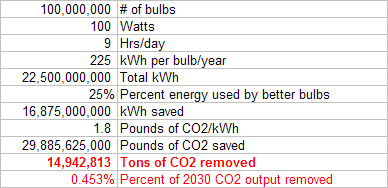
Replacing 100 million light bulbs with more efficient compact fluorescent or LED bulbs could save 15 million tons of CO2.
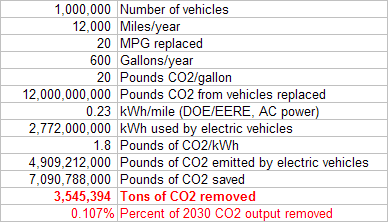
A shocking finding for me was how little CO2 would be prevented by putting a million EVs on the road. Granted, part of this is because right now a big chunk of electricity comes from burning coal. If we moved more of our electricity generation to renewables, this number might increase. But right now a million EVs would remove less than 4 million tons of CO2, the same as 200 tons of iron fertilization.
On this rough evidence, OIF is definitely worth further investigation.


